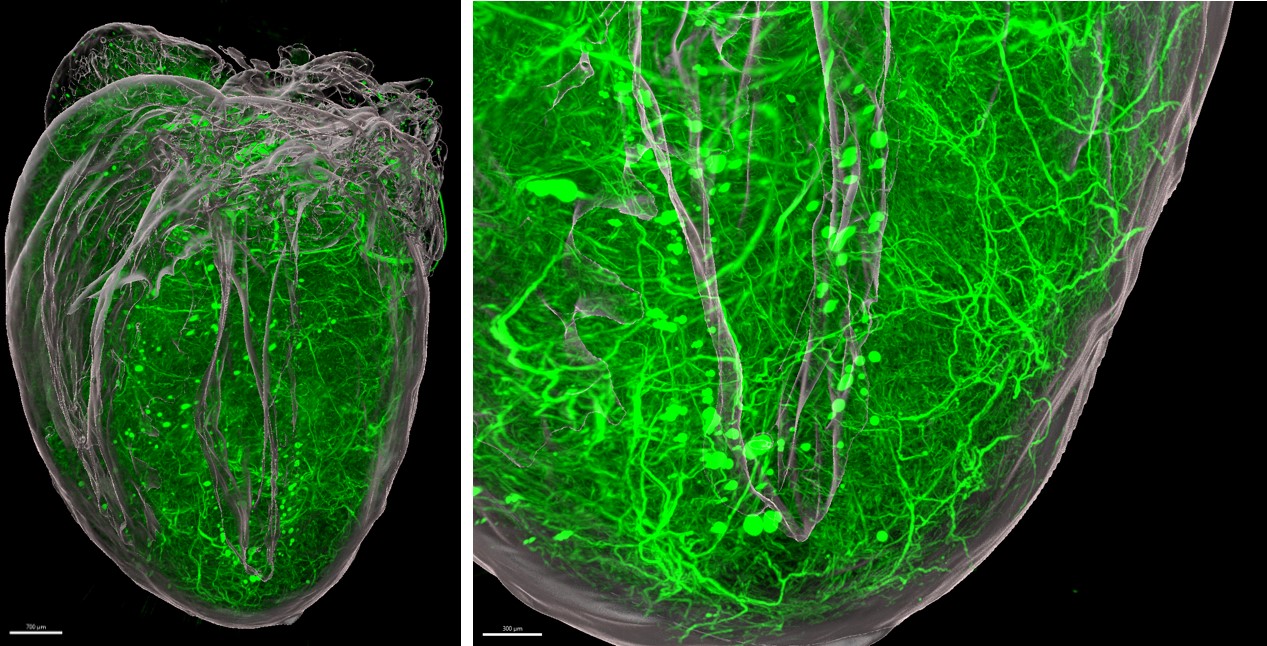
Cardiac innervation modulates arrhythmogenesis
Team

PD Dr. med.
Christiane Jungen

Hunar
Haydar
Medizinischer Doktorand

Dr. rer. nat.
Yanis Mouloud
Topics and Vision
Cardiovascular diseases are up to now the most important cause of morbidity and mortality. Cardiac autonomic nervous system dysfunction is associated with most cardiovascular diseases and can initiate and exacerbate heart disease. Pathological changes in the cardiac autonomic nervous system predispose to potentially lethal heart rhythms such as ventricular arrhythmias. Only recently it was discovered that cardiac inflammation may also be relevant for the initiation and perpetuation of arrhythmias.
The research group focuses on identifying mechanisms underlying ventricular arrhythmogenesis with regard to neuronal remodelling and cardiac inflammation. The goal is to reduce arrhythmogenesis by exploring new therapeutical approaches.
The translational research uses state-of-the-art methodology with direct clinical relevance. Preceding work of the CardioScienceLabs on myocardial immune regulation and advanced cardiac imaging lays the basis for the current projects connecting neuro-cardiology with cardiac inflammation.
Projects
- Imaging of the cardiac autonomic nervous system simultaneously with cardiac inflammation using light sheet fluorescence microscopy
- Interplay of the autonomic nervous system with cardiac inflammation and its relevance for ventricular arrhythmogenesis
- DFG: Relevance of sympathetic innervation and myocardial substrate for ventricular arrhythmogenesis in patients with non-ischemic cardiomyopathy

Methods
- Models
- In-vivo ischaemia/reperfusion model
- Depletion of sympathetic fibers and
- Isolated mouse hearts (Ex-vivo Langendorff)
- Imaging
- Light microscopy
- Confocal microscopy
- Lightsheet fluorescence microscope
- Molecular
- Western blot
- PCR, Real-time PCR
- Cytokine array
- Various ELISAs
- Flow cytometry
- Translational
- Patient samples (pending)
- Clinical data
Funding & Awards
- 2020 – 2023: DFG Walter-Benjamin Stipendium at Willem Einthoven Center for Cardiac Arrhythmia Research & Management, University Clinic Leiden, The Netherlands (Prof. Zeppenfeld)
- 04/2018: Poster Award, German Society of Cardiology
- 03/2017: Paper of the Month German Center for Cardiovascular Research (DZHK): Nature Communications: Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias.
- 11/2016: Promotionspreis der Landesärztekammer Hessen 2015: Beste Promotion der drei hessischen Universitäten
- 2015: German Center for Cardiovascular Research (DZHK) Travelgrant: Visiting Scientist at the Cardiac Arrhythmia Center of the University of Californa (UCLA), Los Angeles, USA (Prof. Shivkumar)
Publications
- Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, Alken F, Angendohr S, Schmitt C, Mester J, Klöcker N, Veldkamp MW, Schumacher U, Willems S, Nikolaev VO, Meyer C. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nature Communications, 2017; 8:14155.
- Jungen C, Scherschel K, Bork NI, Kuklik P, Eickholt C, Kniep H, Klatt N, Willems S, Nikolaev VO, Meyer C. Studying the impact of intracardiac neurons on cardiac electrophysiology and arrhythmogenesis in an ex-vivo Langendorff system. Journal of Visualized Experiments, 2018 May 22; (135).
- Jungen C, Scherschel K, Flennner F, Jee H, Rajendran P, De Jong K, Nikolaev VO, Meyer C, Ardell J, Tompkins J. Increased arrhythmia susceptibility in type 2 diabetic mice related to dysregulation of ventricular sympathetic innervation. American Journal of Physiology, 2019 Dec 1;317(6):H1328-H1341.
- Scherschel K, Hedenus K, Jungen C, Lemoine MD, Ruebsamen N, Veldkamp MW, Klatt N, Lindner D, Westermann D, Casini S, Kuklik P, Eickholt C, Klöcker N, Shivkumar K, Christ T, Zeller T, Willems S, Meyer C. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Science Translational Medicine. 2019 May 22;11(493).
- Berisha F, Götz K, Wegener JW, Brandenburg S, Subramanian H, Molina CE, Rueffer A, Petersen J, Bernhardt A, Girdauskas E, Jungen C, Pape U, Kraft AE, Warnke S, Lindner D, Westermann D, Blankenberg S, Meyer C, Hasenfuß G, Lehnart SE, Nikolaev VO. cAMP Imaging at Ryanodine Receptors Reveals β2-Adrenoceptor Driven Arrhythmias. Circ Res. 2021 Jun 25;129(1):81-94.